Performance Specifications [17][21][76] *
* Refer "Clinical Data" page - References section
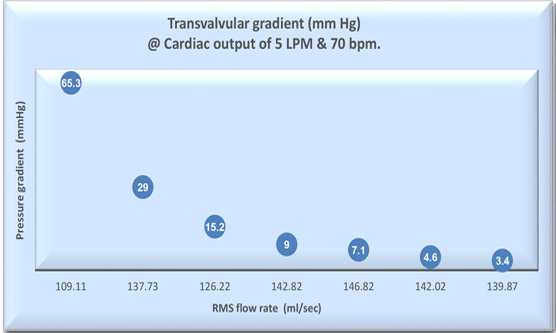
TRANSVALVULAR GRADIENT
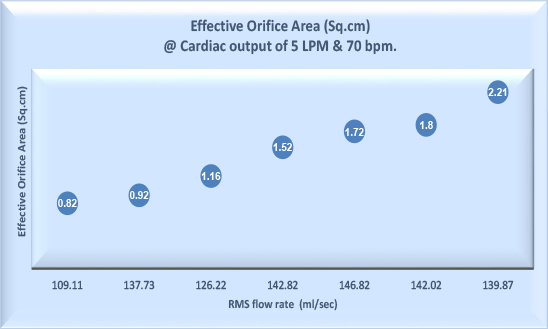
Hydrodynamic testing of the heart valve provides information on the fluid mechanical performance of the heart valve substitute in terms of load to the heart as per EN ISO 5840. Valve performance parameters include
- Pressure differences across the valve
- Effective orifice area

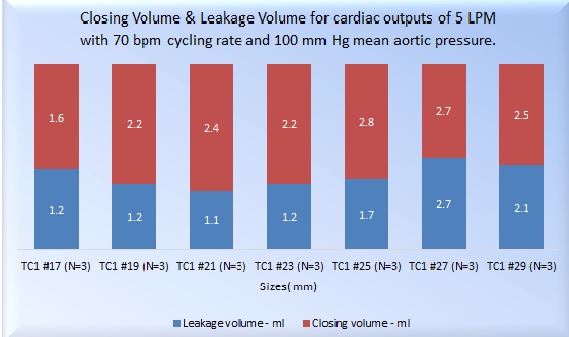
REGURGITATION CHARACTERISTICS
Valve performance parameters estimated during the testing include the average static leakage volume flow rate with the valve in the closed condition against different back pressures
The Operational Specification
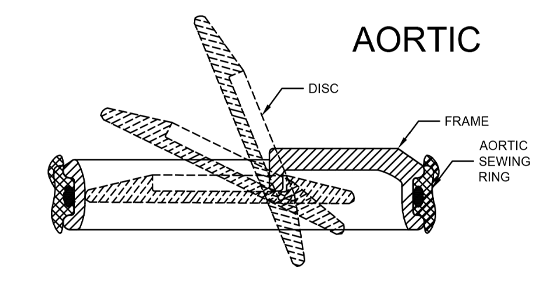
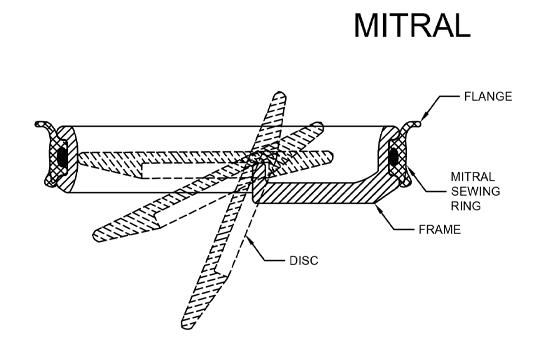
PRINCIPLE OF OPERATION
By means of three metal supports (Struts), the metal ring (Frame) holds the Disc which opens and closes as the heart pumps blood through the valve and thus replicates the function of the natural valves of heart
The three main components of TTK Chitra Heart Valve are:.
- Frame
- Disc
- Sewing Ring
The valve incorporates a Tilting Disc, pivoted eccentrically in the metallic frame. The sewing ring is fitted snugly around the frame and is used to suture the valve in the intended position in the heart. The frame and the disc are hydro-dynamically designed to reduce drag and inertia and polished to minimize the chances of clotting.
DEVICE LIFETIME [21] *
The structural life of the device is estimated as 50 years. This is validated through accelerated durability testing conducted for the 400 million cycles meeting EN ISO 5840.
* Refer "Clinical Data" page - References section
SHELF LIFE
The sterile shelf life of the TTK Chitra Heart Valve Prosthesis, Model TC1/TC1-H is 5 years; this is validated through Accelerated and real time ageing studies.
MATERIAL CHARACTERISATION & BIOCOMPATIBILITY

Material properties of all constituent materials comprising the surgical heart valve substitute is evaluated as applicable to specific design as per EN ISO 5840. The biocompatibility of the materials and components used in heart valve substitutes meets the requirements of IS/ISO 10993 & EN ISO 10993.
SALIENT FEATURES